【论著】| 长链非编码RNA FLJ30679对口腔鳞状细胞癌细胞增殖和迁移的影响
时间:2024-06-16 06:00:55 热度:37.1℃ 作者:网络
[摘要] 背景与目的:长链非编码RNA(long noncoding RNA,lncRNA)能够调控基因转录、mRNA剪切、稳定和翻译,是多种生物学过程中的重要调节因子。本研究旨在探讨lncRNA FLJ30679在口腔鳞状细胞癌(oral squamous cell carcinoma,OSCC)中的表达、与临床病理学特征的关系及对OSCC恶性生物学行为的影响。方法:通过UCSC Xena数据库分析FLJ30679在头颈部鳞状细胞癌(head and neck squamous cell carcinoma,HNSCC)组织中的表达及与临床病理学特征的关系。通过实时荧光定量聚合酶链反应(real-time fluorescence quantitative polymerase chain reaction,RTFQ-PCR)检测FLJ30679在OSCC细胞系中的表达,通过RNA核质分离实验明确其亚细胞定位;采用FLJ30679 Smart Silencer建立FLJ30679敲减组(SS-FLJ30679),采用过表达质粒建立FLJ30679过表达组(FLJ30679)。通过细胞计数试剂盒-8(cell counting kit-8,CCK-8)和transwell迁移实验检测FLJ30679表达改变对OSCC细胞增殖和迁移能力的影响。通过RTFQ-PCR和蛋白质印迹法(Western blot)检测OSCC细胞中的FLJ30679表达改变对上皮-间充质转化(epithelial-mesenchymal transition,EMT)相关基因表达的影响。通过Western blot检测FLJ30679表达改变对磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/蛋白激酶B(protein kinase,AKT)通路的影响。结果:数据库分析显示,FLJ30679在HNSCC组织中的表达高于正常组织 (P<0.01),且其表达与不良预后有关(P<0.01)。FLJ30679在6种OSCC细胞系中均高表达,且主要定位于细胞核。与对照组相比,FLJ30679敲减组的OSCC细胞增殖和迁移能力显著降低(P<0.01),FLJ30679过表达组的OSCC细胞增殖和迁移能力显著升高(P<0.001)。FLJ30679敲减导致E-钙黏蛋白(E-cadherin)的mRNA和蛋白表达水平上调(P<0.01),而N-钙黏蛋白(N-cadherin)和波形蛋白(vimentin)的mRNA和蛋白表达水平下调(P<0.01);FLJ30679过表达导致E-cadherin的蛋白表达水平下调(P<0.001),而N-cadherin和vimentin的mRNA和蛋白表达水平上调(P<0.05)。FLJ30679敲减导致磷酸化PI3K(phosphorylated-PI3K,p-PI3K)和磷酸化AKT(phosphorylated-AKT,p-AKT)的蛋白表达水平下调(P<0.001),FLJ30679过表达导致p-PI3K和p-AKT的蛋白表达水平上调(P<0.01)。结论:FLJ30679在OSCC细胞和组织中表达上调,能够促进OSCC细胞增殖和迁移,这可能与FLJ30679激活PI3K/AKT通路,促进EMT的发生有关。
[关键词] 口腔鳞状细胞癌;长链非编码RNA;FLJ30679;细胞增殖;细胞迁移;上皮-间充质转化
[Abstract] Background and purpose: Long noncoding RNA (lncRNA) can regulate gene transcription, mRNA shear, stabilization and translation, and it is an important regulatory factor in a variety of biological processes. This study aimed to investigate the expression and clinical features of lncRNA FLJ30679 in oral squamous cell carcinoma (OSCC) and its effect on the malignant biological behavior of OSCC. Methods: The expression of FLJ30679 in head and neck squamous cell carcinoma (HNSCC) tissues and normal tissues was analyzed by the UCSC Xena database for expression and prognosis. The expression of FLJ30679 in OSCC cell lines was detected by real-time fluorescence quantitative polymerase chain reaction (RTFQ-PCR). The subcellular localization of FLJ30679 in OSCC cells was detected by RNA nuclear-cytoplasmic fractionation assays. FLJ30679 Smart Silencer was used to establish the FLJ30679 knockdown group (SS-FLJ30679), and overexpression plasmid of FLJ30679 was used to establish FLJ30679 overexpression group (FLJ30679). The effects of altered FLJ30679 expression on the proliferative and migration capacity of OSCC cells were examined by cell counting kit-8 (CCK-8) and transwell migration assays. RTFQ-PCR and Western blot were used to determine the effect of altered FLJ30679 expression on the expression of epithelial-mesenchymal transition (EMT)- related genes in OSCC cells. The effects of altered FLJ30679 expression on the phosphoinositide 3-kinase (PI3K)/protein kinase (AKT) pathway were detected by Western blot. Results: Online query of database showed that FLJ30679 expression was higher in HNSCC tissues compared to normal tissues (P<0.01). HNSCC patients with higher FLJ30679 expression had lower overall survival (P<0.01). The RTFQ-PCR results showed that FLJ30679 was expressed at a higher level in six OSCC cell lines compared with normal cells, and was predominantly localized in the nucleus. The ability of OSCC cells in the SS-FLJ30679 group to proliferate and migrate was significantly lower compared with the SS-NC group (P<0.01). OSCC cells in the FLJ30679 overexpression group had significantly higher proliferative and migratory capacities than those in the vector group (P<0.001). RTFQ-PCR and Western blot results showed that FLJ30679 knockdown resulted in upregulation of mRNA and protein expression levels of E-cadherin (P<0.01) and downregulation of mRNA and protein expression levels of N-cadherin and vimentin (P<0.01). FLJ30679 overexpression resulted in downregulation of protein expression levels of E-cadherin (P<0.01) and upregulation of mRNA and protein expression levels of N-cadherin and vimentin (P<0.05). Western blot results showed that knockdown of FLJ30679 resulted in decreased protein expression levels of phosphorylated-PI3K (p-PI3K) and phosphorylated-AKT (p-AKT) (P<0.001), and overexpression of FLJ30679 resulted in increased protein expression levels of p-PI3K and p-AKT (P<0.01). Conclusion: The expression of FLJ30679 was increased in OSCC tissues and cells. It promoted the proliferation and migration ability of OSCC cells, which may be caused by FLJ30679 promoting EMT via PI3K/AKT pathway.
[Key words] Oral squamous cell carcinoma; Long noncoding RNA; FLJ30679; Cell proliferation; Cell migration; Epithelial- mesenchymal transition
口腔鳞状细胞癌(oral squamous cell carcinoma,OSCC)是头颈部鳞状细胞癌(head and neck squamous cell carcinoma,HNSCC)中的最常见类型[1]。由于OSCC患者早期临床特征少且不明显,确诊时往往已处于晚期[2]。目前主要治疗方法仍是手术、化疗、放疗或综合治疗,虽然治疗方法在持续改善,但OSCC患者的预后仍较差[3-4]。因此,确立早期诊断OSCC的策略仍十分重要。
长链非编码RNA(long noncoding RNA,lncRNA)是长度大于200个核苷酸的链式RNA,是癌症中常见的基因表达调控因子[5]。lncRNA可由蛋白质编码基因之间、内含子、天然反义转录本或从不同的增强子和启动子转录而来[6],能够调控基因的转录、mRNA剪切、稳定和翻译等,是多种生物学过程的重要调节因子[7]。通过查询癌症基因组图谱(The Cancer Genome Atlas,TCGA)数据库发现,lncRNA FLJ30679在HNSCC组织内表达上调,其位于染色体16q24.1,由1个外显子组成,长度为1 980 bp。FLJ30679与肺癌的不良预后有关[8],且其高表达往往与肺腺癌患者的低生存率有关[9]。目前关于FLJ30679在OSCC中的作用机制尚不清楚。本研究旨在探讨FLJ30679在OSCC细胞中的表达和定位,以及其表达改变后对OSCC细胞增殖和迁移的影响,旨在为OSCC的分子机制、诊治分子靶标的研究提供新思路。
1 材料和方法
1.1 材料
DMEM和DMEM/F12(1∶1)培养基均购自上海源培生物科技股份有限公司,胎牛血清(fetal bovine serum,FBS)购自赛澳美细胞技术(北京)有限公司,青霉素-链霉素、无血清细胞冻存液、0.25%乙二胺四乙酸(ethylene diamine tetraacetic acid,EDTA)胰蛋白酶消化液、超敏电化学发光(electro chemical luminescence,ECL)试剂盒和细胞计数试剂盒-8(cell counting kit-8,CCK-8)均购自苏州新赛美生物科技有限公司,磷酸缓冲盐溶液(phosphate-buffered saline,PBS)购自天津灏洋生物科技有限公司,反转录试剂盒、实时荧光定量聚合酶链反应(real-time fluorescence quantitative polymerase chain reaction,RTFQ-PCR)试剂盒均购自湖南艾科瑞生物工程有限公司,TRIzol试剂、LipofectamineTM3000转染试剂和PARISTM Kit均购自美国Thermo Fisher Scientific公司,E-钙黏蛋白(E-cadherin)、N-钙黏蛋白(N-cadherin)、波形蛋白(vimentin)和β-actin抗体均购自武汉赛维尔生物科技有限公司,磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)、蛋白激酶B(protein kinase,AKT)、磷酸化PI3K(phosphorylated-PI3K,p-PI3K)(Tyr458)/(Tyr199)和磷酸化AKT(phosphorylated-AKT,p-AKT)(Ser473)抗体均购自美国Cell Signaling Technology公司,HRP标记的羊抗兔二抗购自合肥兰杰柯科技有限公司。
1.2 实验方法
1.2.1 数据库在线分析
使用UCSC Xena数据库(http://xena.ucsc. edu/)[10]的TCGA数据分析FLJ30679在HNSCC组织中的表达和临床意义。纳入分析的标准为:① 原发肿瘤;② 18岁<年龄<80岁。排除分析的标准为:① 有任何其他的肿瘤病史或合并其他肿瘤、转移性肿瘤;② 临床数据不完整或拒绝随访;③ 无准确的FLJ30679表达检测结果。以表达量的中位数为临界值分为FLJ30679High组和FLJ30679Low组。
1.2.2 细胞培养
人OSCC细胞系(HN6、HN30、CAL-27、SCC-9、SCC-4和SCC-25)和正常对照细胞同以往研究[11]。HN6、HN30和CAL-27细胞在含有10%FBS和1%青霉素-链霉素的DMEM高糖培养基中培养,SCC-9、SCC-4和SCC-25细胞在含10%FBS和1%青霉素-链霉素的DMEM/F12 (1∶1)培养基中培养。细胞生长于37 ℃、CO2体积分数为5%且湿度适宜的培养箱中。
1.2.3 总RNA提取、反转录和RTFQ-PCR
使用TRIzol试剂裂解细胞提取总RNA。使用反转录试剂盒进行反转录反应。使用RTFQ-PCR试剂盒配制20 μL的反应体系,采用两步法对目的基因进行扩增。反应条件为:95 ℃ 预变性30 s;95 ℃ 5 s,60 ℃ 30 s,循环40次。以β-actin为内参,通过2-ΔΔCt计算OSCC细胞中FLJ30679的相对表达量,引物序列见表1。
1.2.4 采用RNA核质分离实验检测FLJ30679的亚细胞定位
使用RNA核质分离试剂盒分离细胞核与细胞质的RNA。经过反转录反应后,采用RTFQ-PCR检测。通过2(总RNA的Ct值-组分RNA的Ct值)×100%计算FLJ30679在细胞质和细胞核中的占比。以U6和GAPDH分别作为细胞核和细胞质的内参,判断FLJ30679在细胞质和细胞核中的占比,引物序列见表1。

1.2.5 细胞转染及效率验证
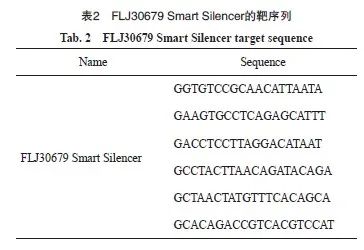
由广州锐博生物科技有限公司设计合成FLJ30679 Smart Silencer,分别为FLJ30679敲减组(SS-FLJ30679)和敲减对照组(SS-NC),靶序列见表2。由上海吉凯基因科技有限公司构建FLJ30679的过表达质粒,分别为FLJ30679过表达组(FLJ30679)和过表达对照组(vector)。将处于对数生长期待转染的CAL-27、HN30和HN6细胞按照每孔2×105个均匀铺至6孔板中,放置于细胞培养箱内培养24 h。待6孔板中的细胞融合度达至60%以上时,使用LipofectamineTM3000转染试剂配制FLJ30679 Smart Silencer/质粒的转染体系,均匀缓慢滴加到整个孔后轻轻混匀。继续培养48 h后,使用TRIzol试剂裂解细胞提取总RNA,采用RTFQ-PCR检测对于FLJ30679的转染效率。
1.2.6 采用CCK-8实验检测细胞增殖能力
收集FLJ30679 Smart Silencer/质粒转染24 h后的细胞,接种于96孔板(1×103个/孔)中,于细胞培养箱内培养。每天定时加入10 μL/孔的CCK-8,测量于450 nm处的吸光度(D)值,连续检测5 d。
1.2.7 采用transwell迁移实验检测细胞迁移能力
收集FLJ30679 Smart Silencer/质粒转染24 h后的细胞,离心后用无血清细胞培养基重悬,并接种于transwell小室的上室(约2.5×104个/100 μL),将600 μL含10%FBS的培养基加入到小室所处的24孔板下室中,于细胞培养箱内培养48 h;使用4%多聚甲醛溶液固定,0.1%结晶紫染色,棉签擦去上室未穿膜的细胞,在光学显微镜下随机选取5个视野拍照并分析。
1.2.8 采用蛋白质印迹法(Western blot)检测蛋白的表达水平
使用全细胞裂解液裂解细胞提取蛋白质,收集裂解液后于105 ℃金属浴10 min。使用二辛可宁酸(bicinchoninic acid,BCA)蛋白定量试剂盒测定蛋白质浓度,并按照所测蛋白质浓度加入适量5×十二烷基硫酸钠聚丙烯酰胺凝胶电泳(sodium dodecylsulphate polyacrylamide gel electrophoresis,SDS-PAGE)蛋白上样缓冲液;随后制胶进行上样,使用10% SDS-PAGE进行凝胶电泳,冰浴下电转;使用5%脱脂奶粉封闭1 h,在4 ℃摇床上过夜温育一抗[N-cadherin、E-cadherin、vimentin、PI3K、p-PI3K(Tyr458)/(Tyr199)、AKT、p-AKT(Ser473)和β-actin均为1∶1 000];第2天使用含有吐温-20三乙醇胺缓冲盐溶液(tris-buffered saline Tween,TBST)洗膜3次,于室温下温育二抗(1∶10 000),再次使用TBST洗膜3次后,使用ECL显影液通过凝胶成像系统显影;使用Image J软件进行灰度分析。
1.3 统计学处理
采用SPSS 19.0软件及GraphPad 7图形处理软件对数据进行统计分析。计量资料以x±s表示,两组间比较采用Student t检验。多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。对于临床数据的分析,采用Kaplan-Meier法制作生存曲线,以log-rank法比较两组总生存率的差异。采用COX比例风险模型进行单因素和多因素回归分析,以确定影响HNSCC患者生存的独立预后因素。P<0.05为差异有统计学意义。
2 结 果
2.1 FLJ30679在HNSCC组织中的表达和临床意义
UCSC Xena数据库中的TCGA数据的纳入和排除流程图见图1A。通过UCSC Xena数据库中的TCGA泛癌基因表达数据和临床数据分析显示,与正常组织(n=44)相比,FLJ30679在HNSCC组织(n=518)中的表达水平较高(HNSCC组织为-5.289±2.087,正常组织为-6.239±1.930,P<0.01,图1B),进一步生成总体生存曲线发现,FLJ30679高表达的患者预后较差(log-rank P<0.01,图1C),在单因素分析中,运用COX比例风险模型来判断FLJ30679的表达水平、病理学分期、临床分期、组织学分级、年龄、性别及种族与预后的关系,发现FLJ30679的表达水平和病理学分期对预后有影响(P<0.05,图1D),并通过多因素回归分析证实,FLJ30679的表达水平和病理学分期是HNSCC患者的独立预后因素(P<0.05,图1E)。进一步通过ENCORI数据库(https://rnasysu.com/encori/index.php)[12]和GEPIA2数据库(http://gepia2.cancer-pku.cn)[13]在线分析显示,与正常组织相比,FLJ30679在HNSCC组织中的表达水平较高,且FLJ30679高表达的患者预后较差。
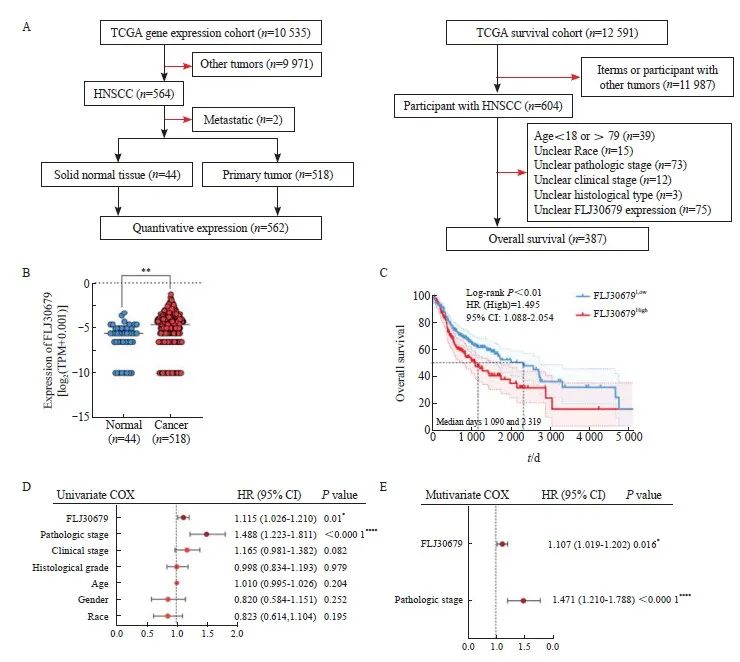
图 1 FLJ30679在HNSCC组织中的表达和预后意义
Fig. 1 Expression and prognostic significance of FLJ30679 in HNSCC tissue
A: Schematic representation of TCGA data inclusion and exclusion in the UCSC Xena database. B: Analysis of FLJ30679 expression in HNSCC and normal tissues in TCGA data (Normal: 44; Cancer: 518). C: Overall survival of patients with high and low FLJ30679 expression, above the median FLJ30679 expression for the FLJ30679High group and below the median FLJ30679 expression for the FLJ30679Low group. D: Univariate COX regression analysis of FLJ30679 expression and other clinical parameters. E: Multivariate COX regression analysis of FLJ30679 expression and other clinical parameters. *: P<0.05, compared with normal. **: P<0.01, compared with normal. ****: P<0.000 1, compared with normal.
2.2 FLJ30679在OSCC细胞中的表达和亚细胞定位
RTFQ-PCR结果显示,FLJ30679在OSCC细胞系中的相对表达量均高于正常细胞(正常细胞为1.000±0.000,HN6细胞为9.524±1.693,HN30细胞为1.556±0.181,CAL-27细胞为4.930±1.207,SCC-4细胞为1.431±0.266,SCC-9细胞为2.662±0.568,SCC-25细胞为2.147±0.078,图2A),将CAL-27、HN6和HN30细胞核质分离后进行RTFQ-PCR,结果显示,FLJ30679主要定位于OSCC细胞核内(以U6、GAPDH作为内参验证分离效果,图2B)。
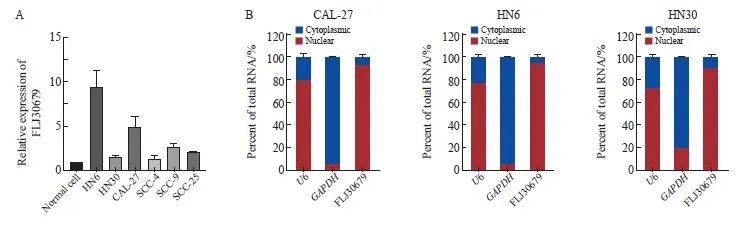
图2 FLJ30679在OSCC细胞系中的表达与亚细胞定位
Fig. 2 Expression and subcellular localization of FLJ30679 in OSCC cell lines
A: The expression of FLJ30679 in OSCC cell lines and normal cells was assessed by RTFQ-PCR. B: RNA nuclear-cytoplasmic fractionation assays were used to detect the subcellular localization of FLJ30679 in CAL-27, HN6 and HN30 cells.
2.3 FLJ30679敲减对OSCC细胞增殖和迁移的影响
RTFQ-PCR结果显示,在CAL-27和HN6细胞中SS-FLJ30679组的FLJ30679相对表达量明显低于SS-NC组(CAL-27细胞:0.441±0.067 vs 1.000±0.000,P<0.001;HN6细胞:0.417±0.011 vs 1.000±0.000,P<0.000 1,图3A)。CCK-8结果显示,SS-FLJ30679组中的CAL-27和HN6细胞增殖能力显著低于SS-NC组(CAL-27细胞的D值:1.895±0.107 vs 2.236±0.043,P<0.000 1;HN6细胞的D值:1.863±0.106 vs 2.403±0.118,P<0.000 1,图3B)。Transwell迁移实验结果显示,SS-FLJ30679组中的CAL-27和HN6细胞迁移能力显著低于SS-NC组(CAL-27细胞迁移数:352.000±23.065 vs 481.000±26.514,P<0.01;HN6细胞迁移数:180.000±23.259 vs 400.667±37.807,P<0.001,图3C)。
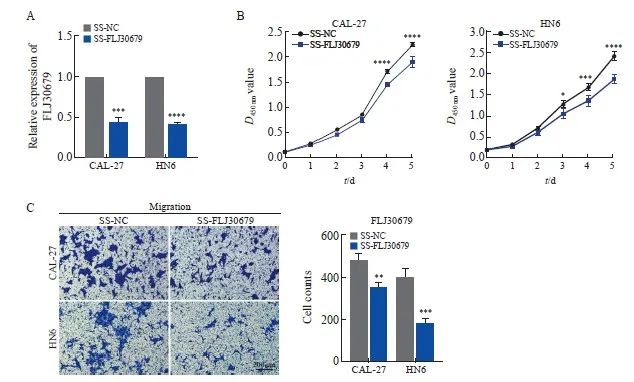
图3 FLJ30679敲减对OSCC细胞增殖和迁移能力的影响
Fig. 3 Effect of FLJ30679 knockdown on proliferation and migration ability of OSCC cells
A: RTFQ-PCR was used to determine the relative expression of FLJ30679 after knockdown of FLJ30679. B: CCK-8 assay was used to determine the effect of FLJ30679 knockdown on the proliferative ability of CAL-27 and HN6 cells. C: Transwell migration assay was used to determine the effect of FLJ30679 knockdown on the migratory ability of CAL-27 and HN6 cells. *: P<0.05, compared with SS-NC; **: P<0.01, compared with SS-NC; ***: P<0.001, compared with SS-NC; ****: P<0.000 1, compared with SS-NC group.
2.4 FLJ30679过表达对OSCC细胞增殖和迁移的影响
RTFQ-PCR结果显示,FLJ30679过表达组的CAL-27和HN30细胞中的FLJ30679相对表达量明显高于vector 组(CAL-27细胞:6 478.826±649.347 vs 1.000±0.000,P<0.000 1;HN30细胞:63 230.811±9 752.614 vs 1.000±0.000,P<0.001,图4A)。CCK-8结果显示,FLJ30679过表达组中的CAL-27和HN30细胞增殖能力显著高于vector组(CAL-27细胞的D值:1.845±0.201 vs 1.228±0.163,P<0.000 1;HN30细胞的D值:2.942±0.051 vs 2.612±0.129,P<0.000 1,图4B)。Transwell迁移实验结果显示,FLJ30679过表达组中的CAL-27和HN30细胞迁移能力显著高于vector组(CAL-27细胞迁移数:638.667±16.623 vs 454.667±24.543,P<0.001;HN30细胞迁移数:219.000±5.568 vs 69.667±6.028,P<0.000 1,图4C)。
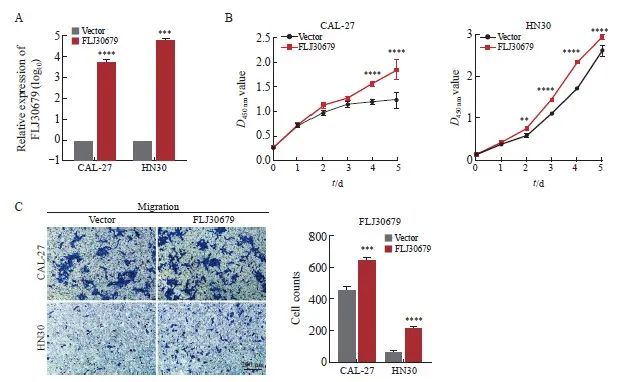
图4 FLJ30679过表达对OSCC细胞增殖和迁移能力的影响
Fig. 4 Effect of FLJ30679 overexpression on proliferation and migration ability of OSCC cells
A: RTFQ-PCR was used to determine the relative expression of FLJ30679 after overexpression of FLJ30679. B: CCK-8 assay was used to determine the effect of FLJ30679 overexpression on the proliferative capacity of CAL-27 and HN30 cells. C: Transwell migration assay was used to determine the effect of FLJ30679 overexpression on the migratory ability of CAL-27 and HN30 cells. **: P<0.01, compared with vector; ***: P<0.001, compared with vector; ****: P<0.000 1, compared with vector group.
2.5 FLJ30679对OSCC细胞上皮-间充质转化(epithelial-mesenchymal transition,EMT)的影响
RTFQ-PCR和Western blot结果显示,与SS-NC组相比,SS-FLJ30679组中的E-cadherin的mRNA水平(CAL-27细胞:1.302±0.064 vs 1.000±0.000,P<0.01;HN6细胞:8.140 ±1.074 vs 1.000±0.000,P<0.001)和蛋白表达水平(CAL-27细胞:1.154±0.070 vs 0.884±0.015,P<0.001;HN6细胞:0.811±0.069 vs 0.606±0.022,P<0.01)均显著升高(图5A、5B);N-cadherin的mRNA水平(CAL-27细胞:0.583±0.108 vs 1.000±0.000,P<0.01;HN6细胞:0.523±0.059 vs 1.000±0.000,P<0.001)和蛋白表达水平(CAL-27细胞:0.749±0.067 vs 1.257±0.101,P<0.000 1;HN6细胞:0.410 ±0.068 vs 0.731±0.096,P<0.001)均显著降低;Vimentin的mRNA水平(CAL-27细胞:0.278±0.158 vs 1.000±0.000,P<0.01;HN6细胞:0.468±0.055 vs 1.000±0.000,P<0.000 1)和蛋白表达水平(CAL-27细胞:0.362±0.060 vs 0.843±0.023,P<0.000 1;HN6细胞:0.392±0.032 vs 0.600±0.058,P<0.01)均显著降低(图5A、5B)。在FLJ30679过表达组中,CAL-27细胞中的E-cadherin的蛋白表达水平(0.486±0.068 vs 0.852±0.039,P<0.000 1)和HN30细胞中的E-cadherin的mRNA水平(0.579±0.147 vs 1.000±0.000,P<0.01)和蛋白表达水平(0.441±0.080 vs 0.726±0.079,P<0.01)均显著低于对照组,而N-cadherin和vimentin的mRNA水平(CAL-27细胞中的N-cadherin:1.918±0.445 vs 1.000±0.000,P<0.05;HN30细胞中的N-cadherin:1.692±0.343 vs 1.000±0.000,P<0.05;CAL-27细胞中的vimentin:1.988±0.353 vs 1.000±0.000,P <0.01;HN30细胞中的vimentin:2.049±0.366 vs 1.000±0.000,P<0.01)和蛋白表达水平(CAL-27细胞中的N-cadherin:1.362±0.077 vs 1.109±0.057,P<0.01;HN30细胞中的N-cadherin:0.914±0.070 vs 0.535±0.076,P<0.000 1;CAL-27细胞中的vimentin:1.136±0.086 vs 0.883±0.031,P<0.01;HN30细胞中的vimentin:0.408±0.041 vs 0.191±0.077,P<0.01)均显著高于对照组(图6A、6B)。
2.6 FLJ30679对OSCC细胞PI3K/AKT通路的影响
Western blot结果显示,与SS-NC组相比,在CAL-27和HN6细胞中SS-FLJ30679组的p-PI3K和p-AKT蛋白表达水平明显较低[CAL-27细胞中的p-PI3K(Tyr458):0.057±0.006 vs 0.201±0.017,P<0.000 1;HN6细胞中的p-PI3K(Tyr458):0.235±0.045 vs 0.468±0.007,P<0.000 1;CAL-27 细胞中的p-PI3K(Tyr199):0.109±0.014 vs 0.241±0.015,P<0.001;HN6细胞中的p-PI3K(Tyr199):0.203±0.004 vs 0.632±0.086,P<0.000 1;CAL-27细胞中的p-AKT(Ser473):0.045±0.014 vs 0.254±0.055,P<0.000 1;HN6细胞中的p-AKT(Ser473):0.054±0.012 vs 0.558±0.042,P<0.000 1],而PI3K/AKT蛋白表达水平无明显差异(P>0.05,图5C)。与vector组相比,在CAL-27和HN30细胞中FLJ30679过表达组的p-PI3K和p-AKT蛋白表达水平明显较高[CAL-27细胞中的p-PI3K(Tyr458):0.607±0.082 vs 0.214±0.034,P<0.000 1;HN30细胞中的p-PI3K(Tyr458):0.298±0.055 vs 0.053±0.032,P<0.001;CAL-27细胞中的p-PI3K(Tyr199):0.360±0.035 vs 0.170±0.013,P<0.000 1;HN30细胞中的p-PI3K(Tyr199):0.253±0.030 vs 0.060±0.002,P<0.01;CAL-27细胞中的p-AKT(Ser473):0.364±0.021 vs 0.153±0.012,P<0.000 1;HN30细胞中的p-AKT(Ser473):0.326±0.026 vs 0.043±0.026,P<0.000 1],而PI3K/AKT蛋白表达水平无明显差异(P>0.05,图6C)。
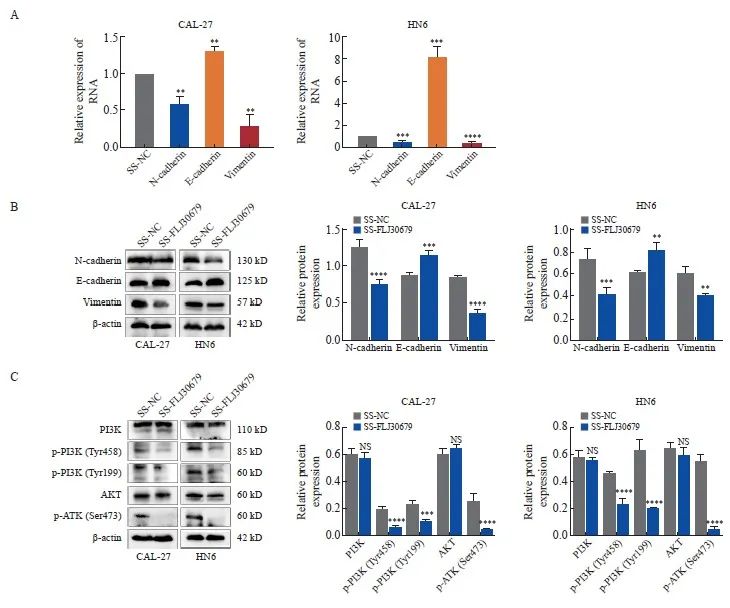
图5 FLJ30679敲减对EMT相关基因与PI3K/AKT通路表达的影响
Fig. 5 Effect of FLJ30679 knockdown on the expression of EMT genes and PI3K/AKT pathway
A: RTFQ-PCR was used to detect the mRNA expression levels of EMT genes after knockdown of FLJ30679. B: Western blot was used to detect theprotein levels of EMT genes after knockdown of FLJ30679. C: Western blot assay used to detect the protein expression levels of PI3K/AKT and p-PI3K/p-AKT after knockdown of FLJ30679. **: P<0.01, compared with vector group or SS-NC group; ***: P<0.001, compared with vector group or SSNC group; ****: P<0.000 1, compared with vector group or SS-NC group; NS: No significance.
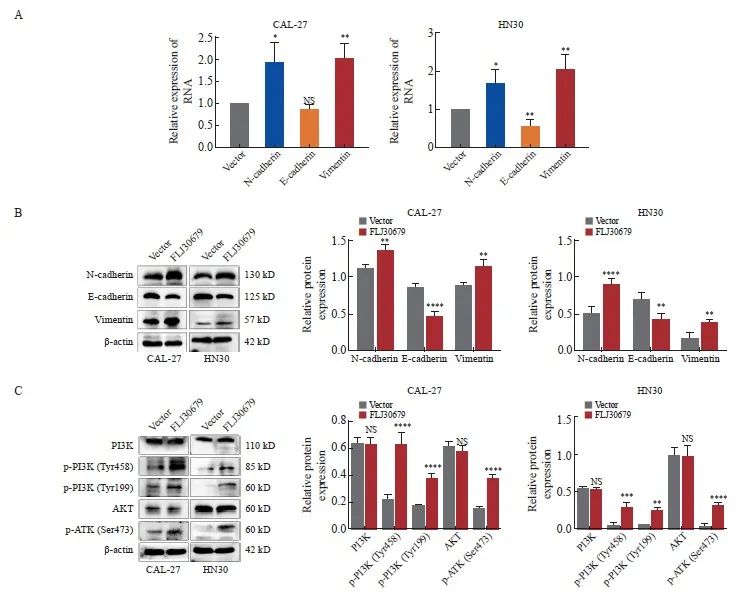
图6 FLJ30679过表达对EMT相关基因与PI3K/AKT通路表达的影响
Fig. 6 Effect of overexpression of FLJ30679 on the expression of EMT genes with PI3K/AKT pathway
A: RTFQ-PCR was used to detect the mRNA expression levels of EMT genes after overexpression of FLJ30679. B: Western blot was used to detect the protein levels of EMT genes after overexpression of FLJ30679. C: Western blot was used to detect the protein expression levels of PI3K/AKT and p-PI3K/p-AKT after overexpression of FLJ30679. *: P<0.05, compared with vector group; **: P<0.01, compared with vector group; ***: P<0.001, compared with vector group; ****: P<0.000 1, compared with vector group; NS: No significance.
3 讨 论
近年来尽管OSCC的诊治取得了很大进展,但是由于OSCC局部复发率和转移率高,5年生存率仍低于50%[14]。lncRNA在OSCC的发生、发展过程中发挥着复杂且精细的调节作用,已有多项研究[15-18]表明,lncRNA行使多种分子功能,参与OSCC细胞增殖、迁移、侵袭、凋亡及免疫逃逸等。其中,OSCC细胞增殖和迁移往往与细胞内高表达的lncRNA有关[19]。由此可知,寻找与OSCC细胞增殖和迁移相关的lncRNA,有助于确定OSCC潜在的生物标志物和治疗靶点,对于提高OSCC患者的生存率具有重要的临床意义。
本研究通过TCGA数据库中大量的基因表达和临床信息分析,发现FLJ30679不仅在OSCC中的表达水平较高,而且FLJ30679表达较高的患者总生存率明显较低,并且通过建立COX比例风险模型,进一步验证了FLJ30679的表达水平和病理学分期可作为OSCC的独立预后因素,对于发现新的OSCC辅助诊断标志物可能具有重要的临床意义。因此本研究选择CAL-27、HN6和HN30细胞作为OSCC的代表性细胞株进行进一步的体外实验,探究FLJ30679在OSCC中的作用。结果显示,FLJ30679敲减能显著抑制OSCC细胞增殖和迁移能力;而FLJ30679过表达能显著促进OSCC细胞增殖和迁移能力,提示FLJ30679在OSCC中发挥着一定的促癌作用。FLJ30679主要定位于细胞核,预示其可能参与基因表达的调控,调节染色质修饰,并与RNA的剪接、修饰和调控稳定性有关[20]。
EMT与肿瘤的发生、侵袭及转移等功能有关[21-22]。EMT是细胞失去上皮特征并获得间充质特征的细胞过程,通过改变细胞形态,改变细胞-细胞和细胞-基质黏附,从而获得更多的侵袭和转移特性[23],EMT也因其与癌症侵袭和转移的密切关系而受到广泛关注[24]。在OSCC中,基因表达可以调节EMT相关基因的表达,影响肿瘤细胞增殖和迁移,促进肿瘤的进展[25]。其中促进间充质标志物vimentin、N-cadherin的表达,以及抑制上皮标志物E-cadherin的表达,对于促进EMT的发生至关重要[26]。本研究发现,FLJ30679敲减可上调E-cadherin的mRNA和蛋白表达水平,下调N-cadherin和vimentin的mRNA和蛋白表达水平;FLJ30679过表达可下调E-cadherin的蛋白表达水平,上调N-cadherin和vimentin的mRNA和蛋白表达水平,从而影响OSCC细胞EMT的发生。PI3K/AKT通路是人类癌症中最常见的激活通路[27],PI3K被酪氨酸激酶激活后,使AKT在细胞膜上聚集并被激活[28]。活化的AKT将磷酸化一系列底物,从而影响多种细胞和生理学过程,包括细胞周期、细胞生长、分化、存活、凋亡、代谢、血管生成和迁移[29]。当PI3K/AKT通路失调时则会影响PI3K/AKT通路的激活[30]。PI3K/AKT通路的激活在调节肿瘤细胞增殖和维持肿瘤细胞的恶性生物学特性中发挥重要作用[31]。Western blot结果表明,FLJ30679可以使PI3K的Tyr199/Tyr458和AKT的Ser473位点发生磷酸化。而已有研究证明,PI3K/AKT通路被激活后可以通过多种方式影响EMT进而影响肿瘤细胞增殖和迁移[32]。本研究证实,FLJ30679敲减可抑制PI3K和AKT蛋白的磷酸化,而FLJ30679过表达可促进PI3K和AKT蛋白的磷酸化,从而影响OSCC细胞中PI3K/AKT通路的激活,提示在OSCC中FLJ30679可能通过激活PI3K/AKT通路来促进EMT的发生,而OSCC细胞迁移能力可能与被促进的EMT进程有关。
综上所述,FLJ30679不仅在OSCC细胞和组织内表达上调,还促进OSCC细胞增殖和迁移能力,这可能是因为FLJ30679通过激活PI3K/AKT通路,促进EMT发生。本研究表明,FLJ30679作为OSCC的致癌因子,在OSCC的发生、发展过程中发挥着重要的促癌作用,可能成为OSCC患者诊断、治疗和预后的新靶点。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:
孙榕婍:文章撰写,实施研究过程;
宋宁:实施研究过程,数据分析;
郑文甜:实施研究过程,图片整理;
张馨月:数据分析,参与文章撰写;
李敏敏:实验分析,文章修改;
公慧:文献调研与整理,文章修改;
蒋英英:设计研究思路及方案,审核文章。
[参考文献]
[1] CHAI A W Y, LIM K P, CHEONG S C. Translational genomics and recent advances in oral squamous cell carcinoma[J]. Semin Cancer Biol, 2020, 61: 71-83.
[2] PANARESE I, AQUINO G, RONCHI A, et al. Oral and oropharyngeal squamous cell carcinoma: prognostic and predictive parameters in the etiopathogenetic route[J]. Expert Rev Anticancer Ther, 2019, 19(2): 105-119.
[3] HE J Y, YE W, KOU N, et al. MicroRNA-29b-3p suppresses oral squamous cell carcinoma cell migration and invasion via IL32/AKT signalling pathway[J]. J Cell Mol Med, 2020, 24(1): 841-849.
[4] KURIHARA-SHIMOMURA M, SASAHIRA T, NAKAMURA H, et al. Zinc finger AN1-type containing 4 is a novel marker for predicting metastasis and poor prognosis in oral squamous cell carcinoma[J]. J Clin Pathol, 2018, 71(5): 436-441.
[5] KOPP F, MENDELL J T. Functional classification and experimental disp of long noncoding RNAs[J]. Cell, 2018, 172(3): 393-407.
[6] ULITSKY I, BARTEL D P. lincRNAs: genomics, evolution, and mechanisms[J]. Cell, 2013, 154(1): 26-46.
[7] GUTTMAN M, RINN J L. Modular regulatory principles of large non-coding RNAs[J]. Nature, 2012, 482(7385): 339-346.
[8] PUDERECKI M, SZUMIŁO J, MARZEC-KOTARSKA B. Novel prognostic molecular markers in lung cancer[J]. Oncol Lett, 2020, 20(1): 9-18.
[9] CHEN M W, LIU B Q, XIAO J B, et al. A novel seven-long non-coding RNA signature predicts survival in early stage lung adenocarcinoma[J]. Oncotarget, 2017, 8(9): 14876-14886.
[10] GOLDMAN M J, CRAFT B, HASTIE M, et al. Visualizing and interpreting cancer genomics data via the Xena platform[J]. Nat Biotechnol, 2020, 38(6): 675-678.
[11] 蒋英英, 陈 曦, 石 雨, 等. 长链非编码RNA COL11A1-208对口腔鳞癌细胞增殖及侵袭的影响[J]. 解
放军医学杂志, 2022, 47(9): 851-862.
JIANG Y Y, CHEN X, SHI Y, et al. Effect of long non-coding RNA COL11A1-208 on proliferation and invasion in oral squamous cell carcinoma cells[J]. Med J Chin People’s Liberation Army, 2022, 47(9): 851-862.
[12] LI J H, LIU S, ZHOU H, et al. starBase v2.0: decoding miRNAceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data[J]. Nucleic Acids Res, 2014, 42(Database issue): D92-D97.
[13] TANG Z F, LI C W, KANG B X, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses[J]. Nucleic Acids Res, 2017, 45(W1): W98-W102.
[14] LI X, WANG C X, ZHANG H, et al. circFNDC3B accelerates vasculature formation and metastasis in oral squamous cell carcinoma[J]. Cancer Res, 2023, 83(9): 1459-1475.
[15] GAO C F, GUO C C, LIU K, et al. LncRNA HOTAIR promotes proliferation and migration of OSCC cells via targeting miR-126 [J]. Minerva Gastroenterol, 2022, 68(2): 245-247.
[16]SHI L J, YANG Y Q, LI M Y, et al. LncRNA IFITM4P promotes immune escape by up-regulating PD-L1 via dual mechanism in oral carcinogenesis[J]. Mol Ther, 2022, 30(4): 1564-1577.
[17]YANG Y X, CHEN D, LIU H, et al. Increased expression of lncRNA CASC9 promotes tumor progression by suppressing autophagy-mediated cell apoptosis via the AKT/mTOR pathway in oral squamous cell carcinoma[J]. Cell Death Dis, 2019, 10(2): 41.
[18]TAO D T, ZHANG Z X, LIU X, et al. LncRNA HOTAIR promotes the invasion and metastasis of oral squamous cell carcinoma through metastasis-associated gene 2[J]. Mol Carcinog, 2020, 59(4): 353-364.
[19]JIANG Y Y, WU K, CAO W, et al. Long noncoding RNA KTN1-AS1 promotes head and neck squamous cell carcinoma cell epithelial-mesenchymal transition by targeting miR-153-3p[J]. Epigenomics, 2020, 12(6): 487-505.
[20]CHEN L L. Linking long noncoding RNA localization and function[J]. Trends Biochem Sci, 2016, 41(9): 761-772.
[21]PASTUSHENKO I, BLANPAIN C. EMT transition states during tumor progression and metastasis[J]. Trends Cell Biol, 2019, 29(3): 212-226.
[22]贾利晴, 葛小路, 姜 琳, 等. LncRNA PKD2-2-3对肺腺癌细胞增殖、克隆形成、迁移及侵袭能力的影响[J]. 中国癌症杂志, 2023, 33(8): 717-725.
JIA L Q, GE X L, JIANG L, et al. Effects of lncRNA PKD2-2-3 on cell proliferation, clone formation, migration, and invasion of lung adenocarcinoma[J]. China Oncology, 2023, 33(8): 717-725.
[23]THIERY J P, ACLOQUE H, HUANG R Y J, et al. Epithelial-mesenchymal transitions in development and disease[J]. Cell, 2009, 139(5): 871-890.
[24]LING Z H, CHENG B, TAO X A. Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: challenges and opportunities[J]. Int J Cancer, 2021, 148(7): 1548-1561.
[25]JIANG Y Y, CAO W, WU K, et al. LncRNA LINC00460 promotes EMT in head and neck squamous cell carcinoma by facilitating peroxiredoxin-1 into the nucleus[J]. J Exp Clin Cancer Res, 2019, 38(1): 365.
[26]YOKOYAMA K, KAMATA N, HAYASHI E, et al. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro[J]. Oral Oncol, 2001, 37(1): 65-71.
[27]HOXHAJ G, MANNING B D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism[J]. Nat Rev Cancer, 2020, 20(2): 74-88.
[28]XU W T, YANG Z, LU N H. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition[J]. Cell Adh Migr, 2015, 9(4): 317-324.
[29]YE B, JIANG L L, XU H T, et al. Expression of PI3K/AKT pathway in gastric cancer and its blockade suppresses tumor growth and metastasis[J]. Int J Immunopathol Pharmacol, 2012, 25(3): 627-636.
[30]FAN Q C, TIAN H, WANG Y, et al. Integrin-α5 promoted the progression of oral squamous cell carcinoma and modulated PI3K/AKT signaling pathway[J]. Arch Oral Biol, 2019, 101: 85-91.
[31]CHAI S L, WEN Z H, ZHANG R X, et al. CCL25/CCR9 interaction promotes the malignant behavior of salivary adenoid cystic carcinoma via the PI3K/AKT signaling pathway[J]. PeerJ, 2022, 10: e13844.
[32]LI Q, LI B, LU C L, et al. LncRNA LINC01857 promotes cell growth and diminishes apoptosis via PI3K/mTOR pathway and EMT process by regulating miR-141-3p/MAP4K4 axis in diffuse large B-cell lymphoma[J]. Cancer Gene Ther, 2021, 28(9): 1046-1057.


