美国在研的乙肝基金仍达64项,这两项特别值得病毒研究者借鉴(2024)
时间:2024-10-06 19:00:56 热度:37.1℃ 作者:网络
乙型肝炎(Hepatitis B)是由乙型肝炎病毒(HBV)引起的一种传染性肝炎,可以导致急性或慢性肝病,慢性乙型肝炎可能导致肝硬化、肝衰竭甚至肝癌。该病毒通过血液、性接触或从母亲传给新生儿的途径传播。
尽管近年来在乙型肝炎的诊断和治疗方面取得了显著进展,但仍存在一些重要而未解决的临床问题:
-
疫苗接种的挑战:虽然有高效的乙型肝炎疫苗,但全球仍有大量未接种疫苗的人群,尤其是在资源有限的国家。
-
治疗耐药性:现有的抗病毒药物虽然可以控制病毒复制和减少肝脏损伤,但耐药性的发展仍是一个重要问题。
-
慢性感染的管理:尽管抗病毒治疗可以有效控制病毒和延缓疾病进展,但目前还没有彻底治愈慢性乙型肝炎的方法。
-
筛查和诊断:在一些地区,由于缺乏资源,乙型肝炎的筛查和早期诊断仍然不足。
-
公共卫生教育和意识提升:提高公众对乙型肝炎传播途径和预防措施的认识是控制这种疾病扩散的关键。
解决这些问题需要全球卫生机构、政府和非政府组织的协作,以提高疫苗接种率,改进抗病毒治疗方案,加强公共健康教育,并推广筛查和早期诊断措施。
我们仅对美国国立卫生研究院(NIH)资助的在研乙型肝炎相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Hepatitis B”为检索词、在题目中进行检索,美国NIH针对乙型肝炎的在研有64项。
一,谁获得了这些研究?
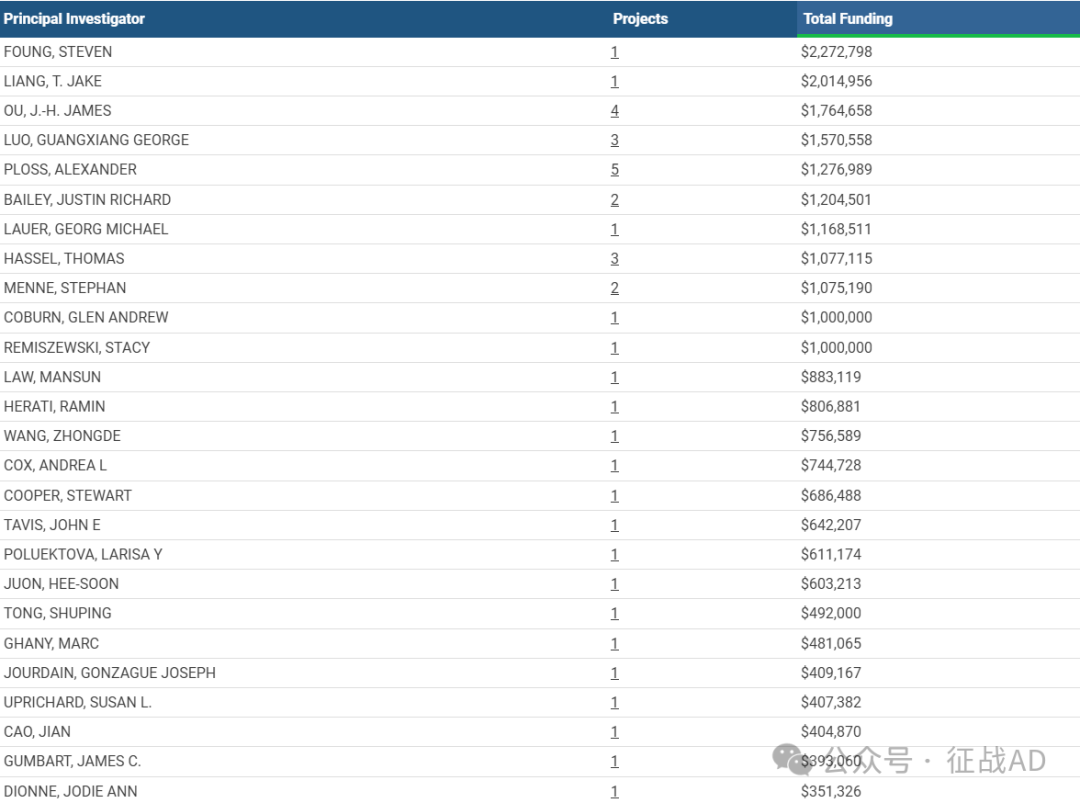
1,在研乙型肝炎基金最多的PI
-
STANFORD UNIVERSITY 的 FOUNG, STEVEN
-
DIABETES, DIGESTIVE, KIDNEY DISEASES 的 LIANG, T. JAKE
-
UNIVERSITY OF SOUTHERN CALIFORNIA 的 OU, J.-H. JAMES
-
WAKE FOREST UNIVERSITY HEALTH SCIENCES 的 LUO, GUANGXIANG GEORGE
-
PRINCETON UNIVERSITY 的 PLOSS, ALEXANDER
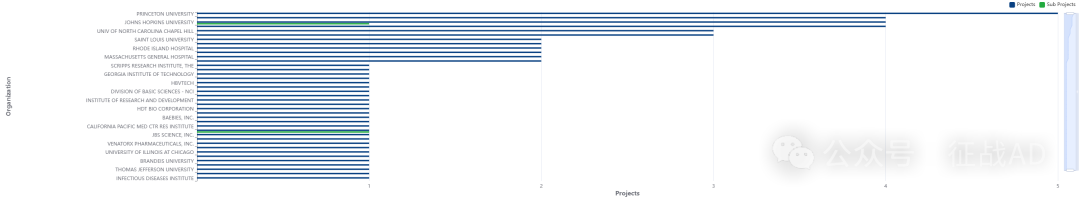
2,乙型肝炎基金最多的研究机构
-
美国国家糖尿病、消化和肾脏疾病研究所
-
斯坦福大学
-
约翰霍普金斯大学
-
南加州大学
-
维克森林大学健康科学系等
二,乙型肝炎研究热点是什么?
乙型肝炎研究领域总览(根据关键词)
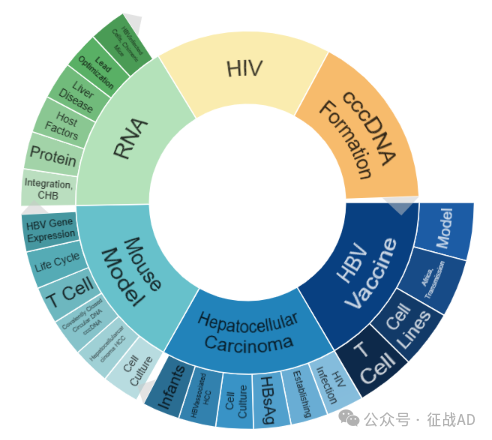
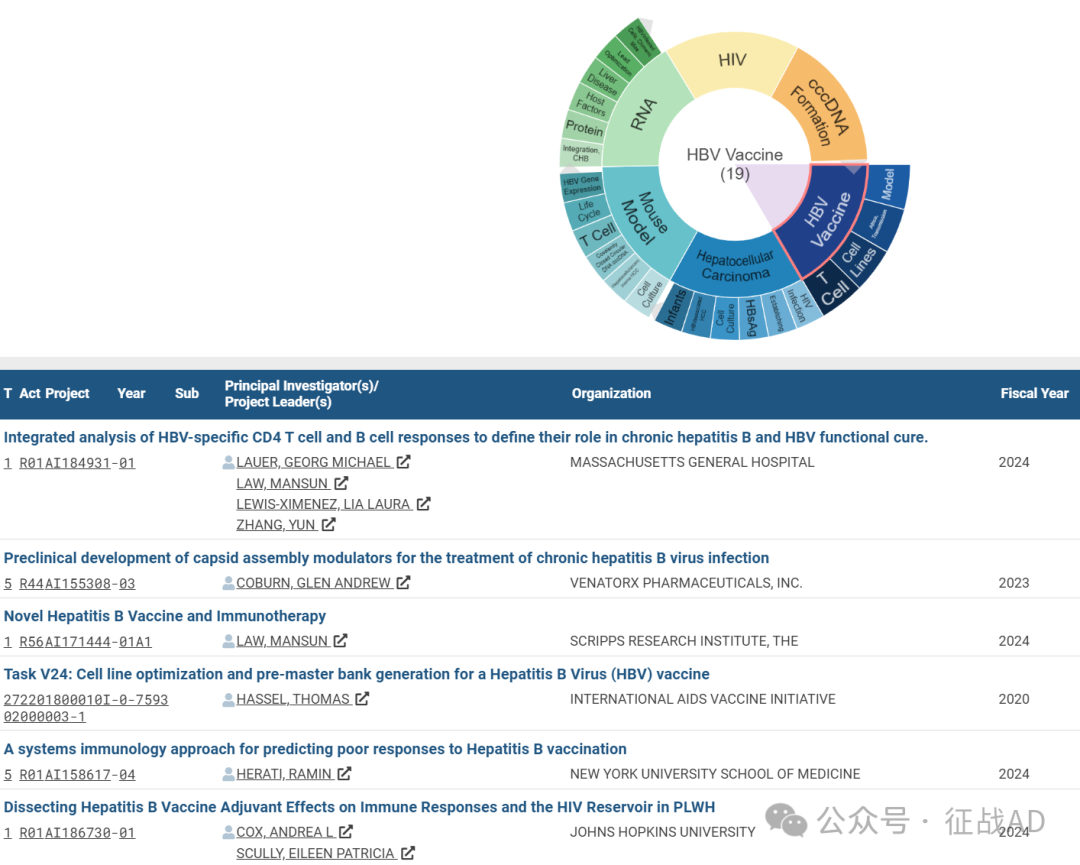
A,关于HBV疫苗(HBV Vaccine)的研究项目最多
有 19 项在研基金涉及到了HBV疫苗,关注最多的方面包括模型(Model)、非洲,传播(Africa, Transmission)、细胞系(Cell Lines)、T细胞(T Cell)等研究。
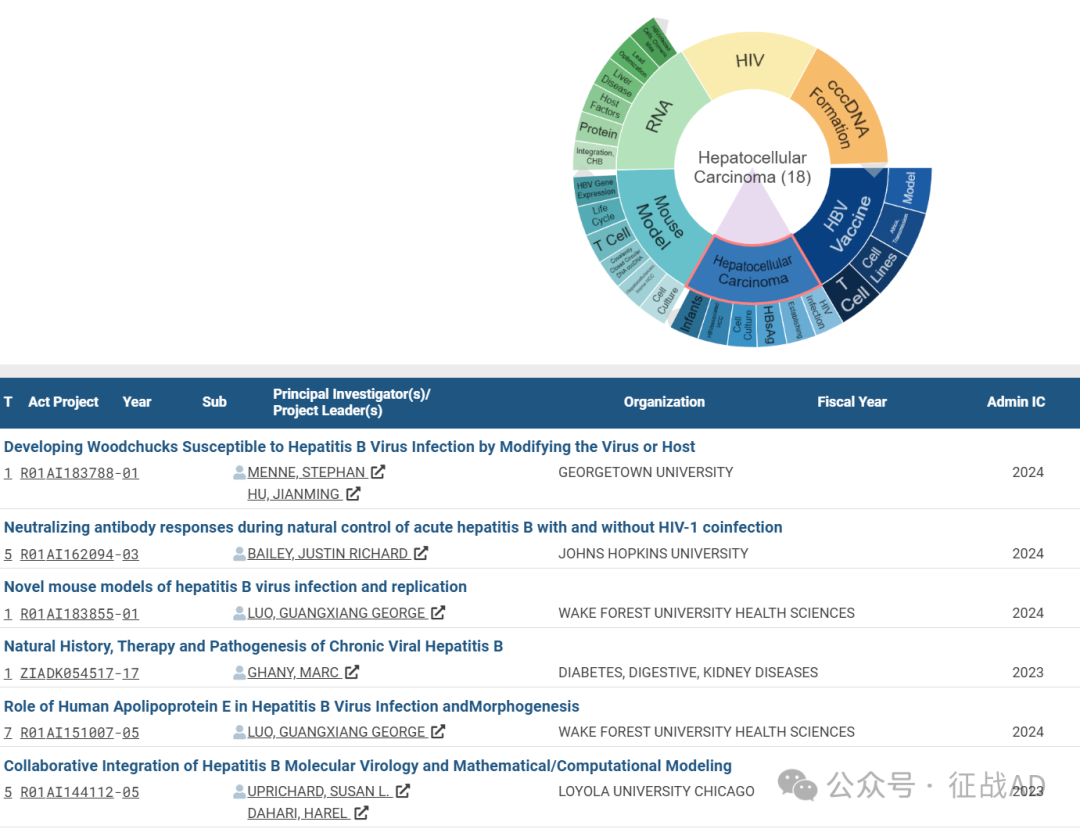
B,关于肝细胞癌(Hepatocellular Carcinoma)的研究项目
有 18 项在研基金涉及到了肝细胞癌,关注最多的方面包括HIV感染(HIV Infection)、建立(Establishing)、乙肝表面抗原(HBsAg)、细胞培养(Cell Culture)、HBV相关肝细胞癌(HBV associated HCC)、婴儿(Infants)等研究。
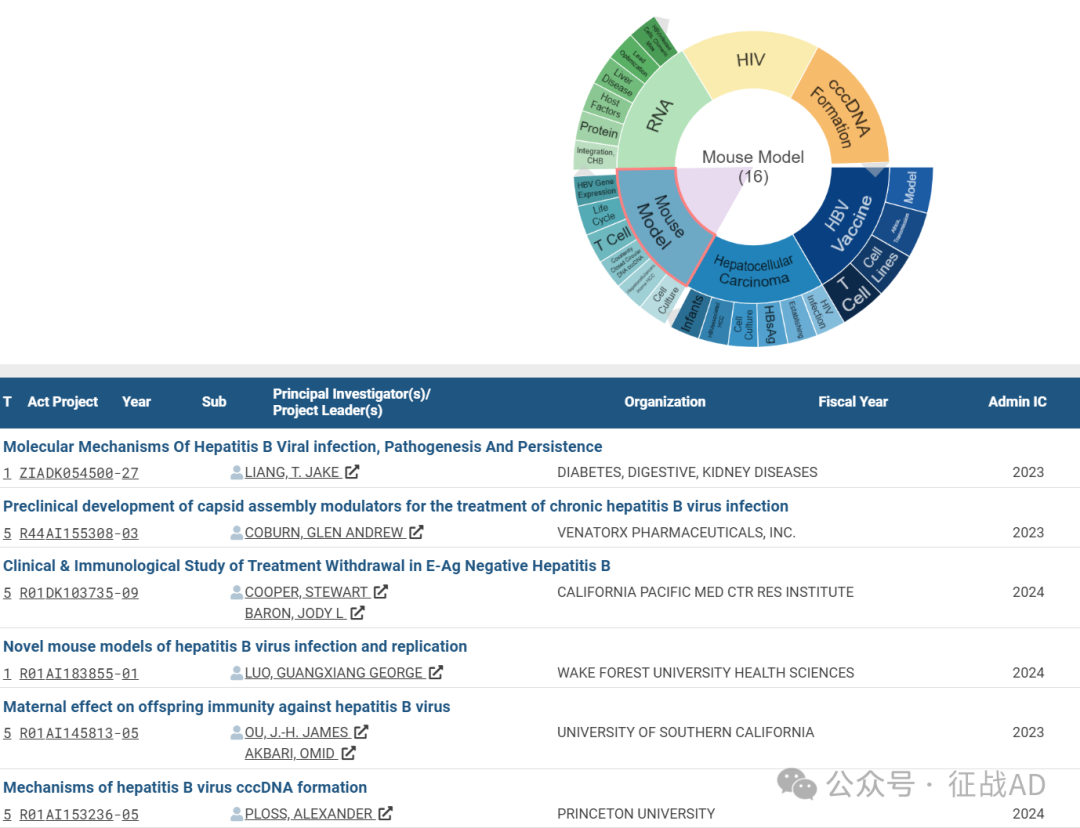
C,关于小鼠模型(Mouse Models)的研究项目
有 16 项在研基金涉及到了小鼠模型,关注最多的方面包括细胞培养(Cell Culture)、T细胞(T Cell)、肝细胞癌(Hepatocellular Carcinoma)、共价闭合环状DNA(Covalently Closed Circular DNA, cccDNA)、T细胞(T Cell)、生命周期(Life Cycle)、HBV基因表达(HBV Gene Expression)等研究。
乙型肝炎研究大的方向还包括HIV、RNA、cccDNA形成(cccDNA Formation)等。
三,借鉴与突破
我们也分享在乙型肝炎领域的几项课题摘要,希望对同仁们有所启发。
A,A vaccine design to induce protective B and T cell immunity against hepatitis C virus
The overall goal of this U19 project is the development of an HCV vaccine to prevent disease progression after virus exposure in a vaccinated host.
This Program is designed to achieve this goal by four complementary Projects and a Scientific Core. Project 1 focuses on a structure-guided approach to develop an immunogen that enhances the induction of broadly neutralizing antibodies (bNAbs) including those when combined lead to synergistic virus neutralization. This project will interact extensively with Project 4 on structural analysis of HCV envelop glycoproteins and on structural aspects of bNAbs and receptor binding to HCV particles. Project 2 will design HCV NS Mosaic antigens for T cell recognition of HCV genotypes and subtypes that cause most infections globally. Mosaic antigens are designed computationally by recombination of viral genomic sequences retrieved from databases. Project 3 takes a systems approach to evaluate short- and long-term B and T cell responses and innate responses to vaccination with vaccines that are formulated in Projects 1 and 3, and in combination with powerful adjuvants.
These studies will be undertaken in non-human primates that will be executed in a Scientific Core. The Administrative Core will provide the operational support necessary to successfully achieved the goals we have laid out for each project and program as a whole.
Taken together, the work proposed in these projects and scientific core are highly interdependent that will lead to a vaccine design capable to elicit protective B and T cell immunity against HCV. We expect that at the end of this program project, we will have a candidate vaccine to begin pre-clinical studies that will progress to Phase I/II clinical trial.
B, Preclinical development of capsid assembly modulators for the treatment of chronic hepatitis B virus infection
One of the most promising classes of compounds under investigation are the core protein allosteric modulators (CpAM) that block HBV replication at multiple stages of the viral life-cycle.
Here, we report on a best-in-class CpAM that exhibits potent pan-genotypic antiviral activity. In this project, we propose to advance our lead CpAM into definitive INDenabling studies to ready the compound for Phase 1 clinical trials in healthy volunteers and chronic HBV patients. Combination regimens that include a CpAM, antivirals possessing distinct mechanisms of action and/or novel immunomodulatory agents will be evaluated in a mouse model of HBV infection in preparation for Phase 2 development in chronic HBV patienst.


