基于病因学分型的研究更受重视。美国在研的47项“周围神经病”基金项目简述(2024)
时间:2024-10-30 06:01:45 热度:37.1℃ 作者:网络
随着对疾病发病机制研究的深入,越来越多的疾病被发现:是具有共同或相似临床表现的一组疾病。
周围神经病(Peripheral Neuropathy)更是如此,这是周围神经系统受损的一组疾病,影响身体远离大脑和脊髓的神经。这些神经负责将信息从大脑和脊髓传递到身体其他部分,以及相反方向的信息传输。当这些神经受损时,可能导致感觉异常、疼痛、肌肉无力或功能障碍。‘
针对周围神经受损机制而进行分类,并由此进一步确认基于发病机制的靶向治疗尤其受到重视。
尽管近年来在周围神经病的诊断和治疗方面取得了显著进展,但仍存在一些重要而未解决的临床问题:
-
诊断挑战:由于周围神经病的症状和原因多样,其诊断可能复杂且困难。有效诊断通常需要通过病史评估、神经功能检查、血液检查、神经电生理检查和有时的神经活检等多种方法综合评估。
-
治疗限制:当前的治疗主要集中在控制症状和治疗根本原因上。对于疼痛的管理,常用的药物如抗抑郁药和抗癫痫药,但这些治疗并不总是有效,且可能有副作用。
-
研究空白:尽管已有一些治疗方法用于减轻症状和延缓疾病进程,但许多类型的周围神经病尚无根治方法。此外,对于神经再生的研究仍处于初级阶段,远未达到临床应用的水平。
-
长期管理:周围神经病患者需要长期、甚至终身的管理和护理。如何提高他们的生活质量,尤其是在严重和持续性症状下,是一个持续存在的问题。
总体而言,周围神经病的治疗和管理需要一个多学科的方法,包括神经学、内科、疼痛管理和康复等专业的合作。未来的研究需要更深入地探索疾病的机制,开发新的治疗方法,以及改进患者的长期照护策略。
我们仅对美国国立卫生研究院(NIH)资助的在研周围神经病相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Peripheral Neuropathy”为检索词、在题目中进行检索,美国NIH针对周围神经病的在研有47项。
一,谁获得了这些研究?
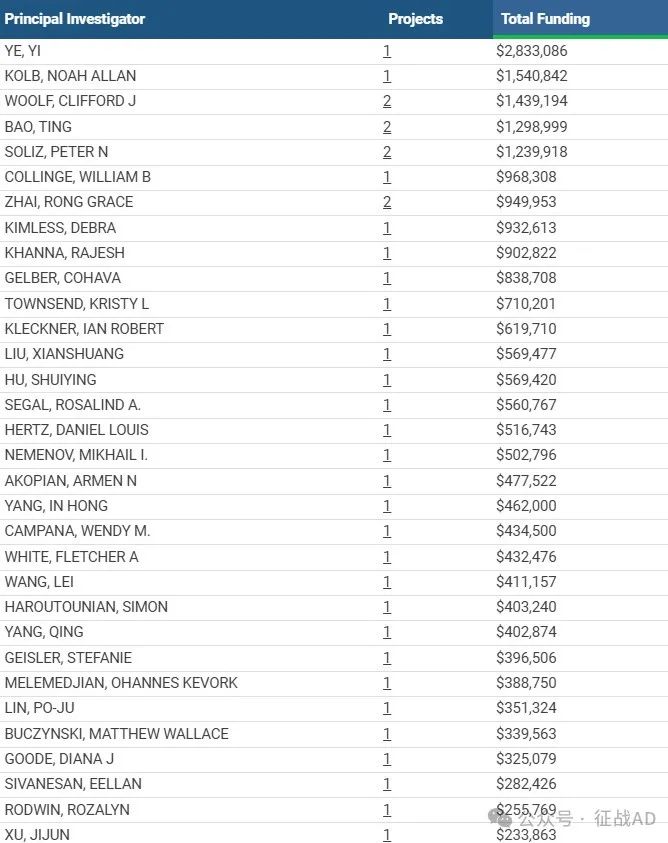
1,在研周围神经病基金最多的PI
-
NEW YORK UNIVERSITY 的 YE, YI
-
UNIVERSITY OF VERMONT & ST AGRIC COLLEGE 的 KOLB, NOAH ALLAN
-
BOSTON CHILDREN'S HOSPITAL 的 WOOLF, CLIFFORD J
-
DANA-FARBER CANCER INST 的 BAO, TING
-
VISIONQUEST BIOMEDICAL INC 的 SOLIZ, PETER N
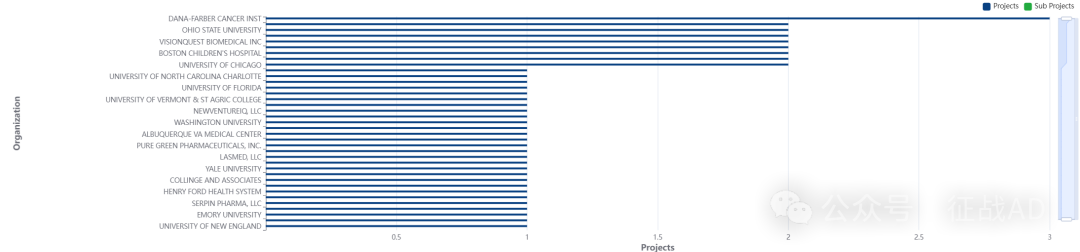
2,周围神经病基金最多的研究机构
-
纽约大学
-
丹娜法伯癌症研究所
-
佛蒙特大学与圣农业学院
-
波士顿儿童医院
-
俄亥俄州立大学等
二,周围神经病研究热点是什么?
周围神经病研究领域总览(根据关键词)
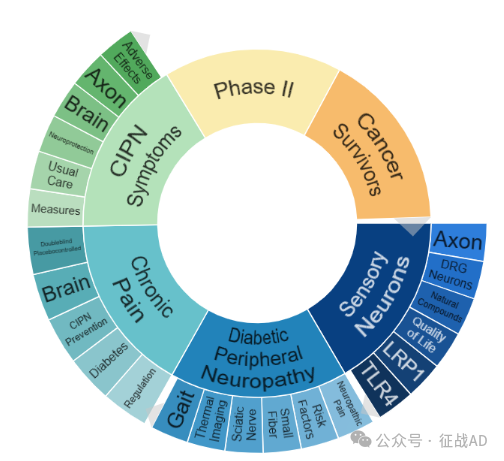
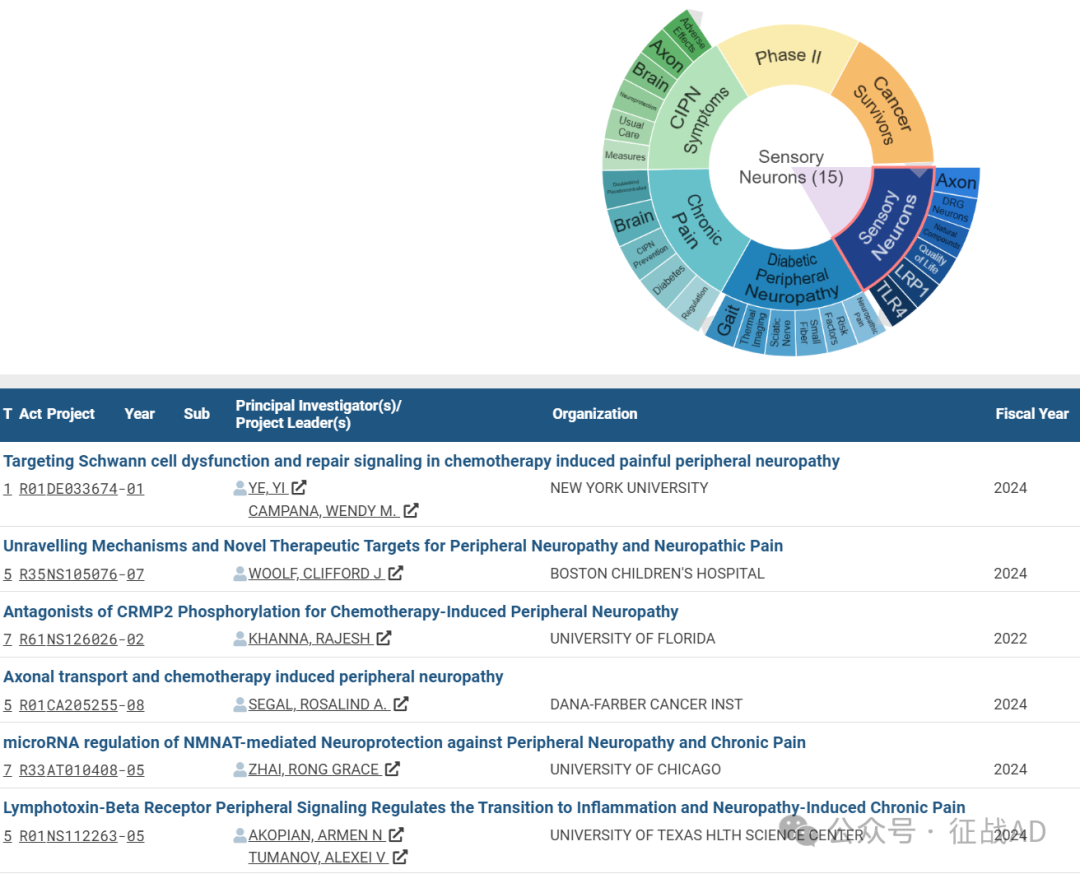
A,关于感觉神经元(Sensory Neurons)的研究项目最多
有 15 项在研基金涉及到了感觉神经元,关注最多的方面包括轴突(Axon)、DRG神经元(DRG Neurons)、天然化合物(Natural Compounds)、生活质量(Quality of Life)、LRP1、TLR4等研究。
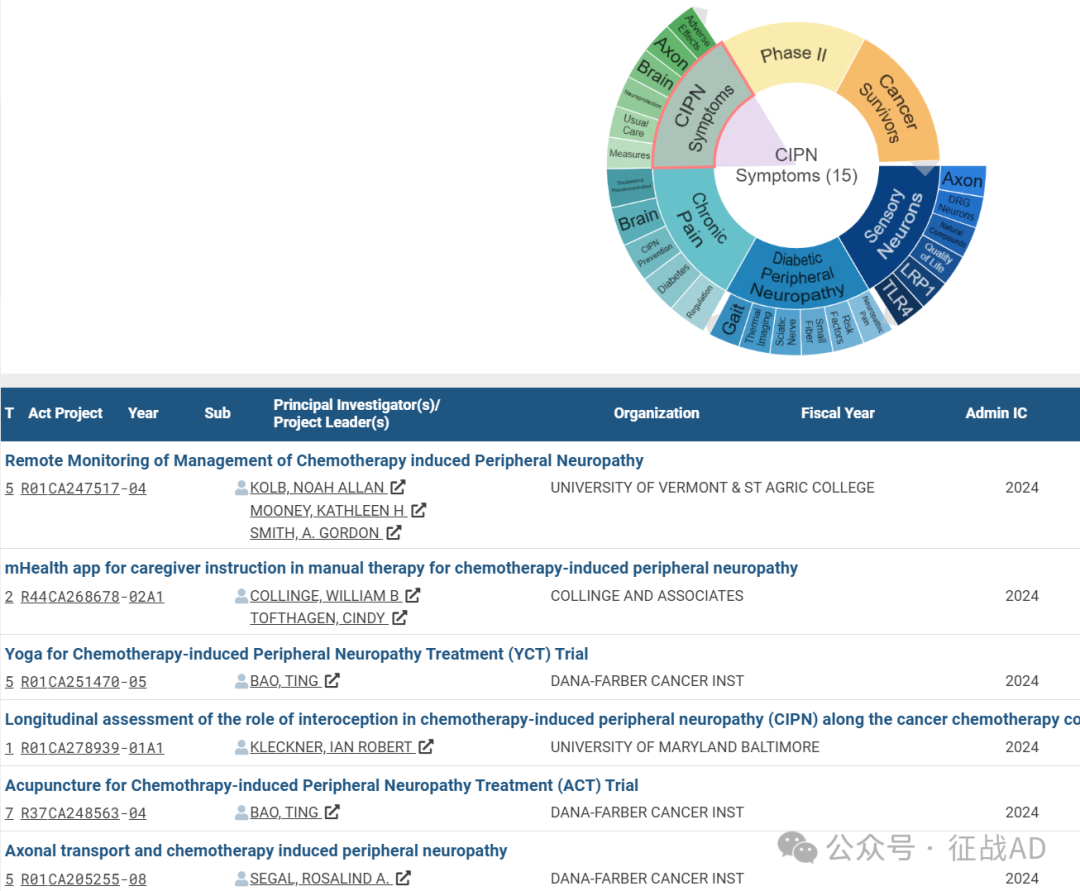
B,关于化疗引起的周围神经病变(CIPN Symptoms)的研究项目
有 15 项在研基金涉及到了化疗引起的周围神经病变,关注最多的方面包括不良反应(Adverse Effects)、轴突(Axon)、大脑(Brain)、神经保护(Neuroprotection)、常规治疗(Usual Care)、措施(Measure)等研究。
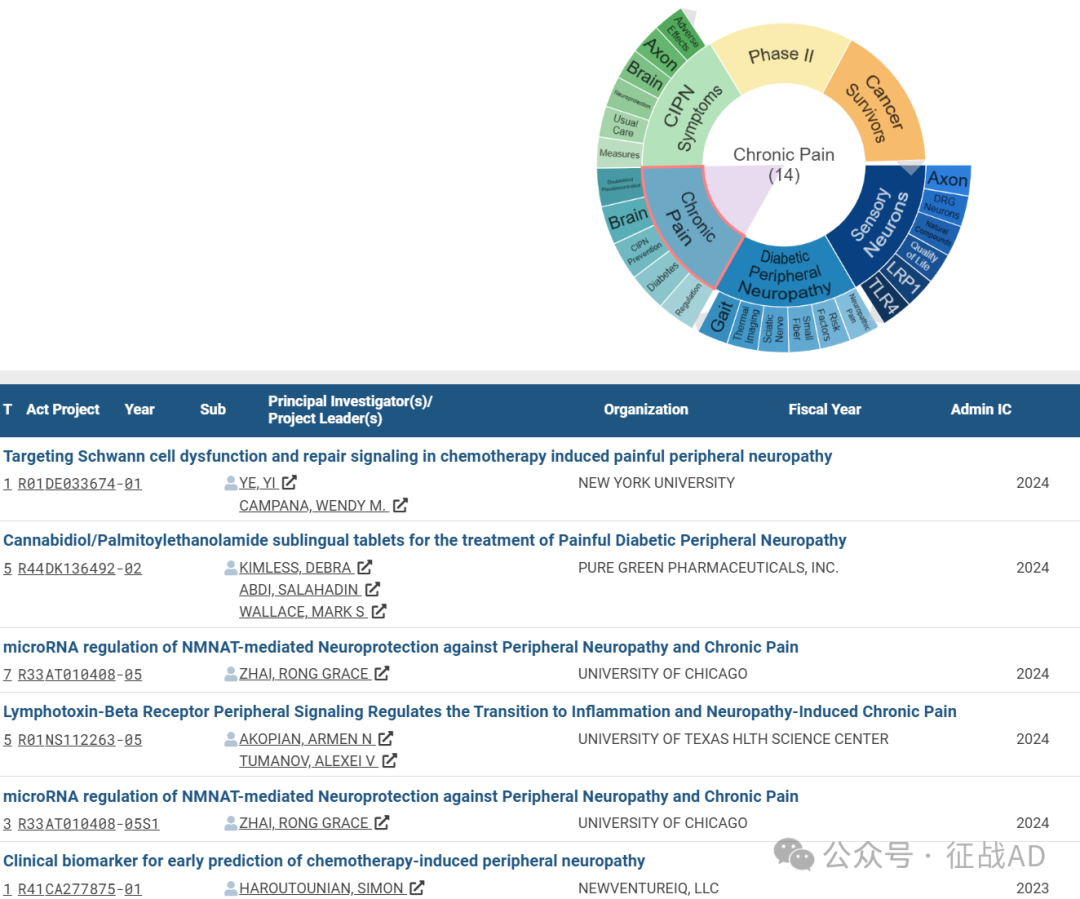
C,关于慢性疼痛(Chronic Pain)的研究项目
有 14 项在研基金涉及到了慢性疼痛,关注最多的方面包括双盲安慰剂对照(Doubleblind Placebocontrolled)、大脑(Brain)、CIPN预防(CIPN Prevention)、糖尿病(Diabetes)、调控(Regulation)等研究。
其他周围神经病研究大的方向包括糖尿病周围神经病变(Diabetic Peripheral Neuropathy)、Phase Ⅱ、癌症幸存者(Cancer Survivors)等。
三,借鉴与突破
我们也分享在周围神经病领域的几项课题摘要,希望对同仁们有所启发。
A,Targeting Schwann cell dysfunction and repair signaling in chemotherapy induced painful peripheral neuropathy
We discovered a key reciprocal signaling system mediated by peroxisome proliferator activated receptor gamma (PPARγ) and low-density lipoprotein receptor related protein 1 (LRP1) that regulate SC survival, bioenergetics and inflammation. We hypothesize that PPARγ and LRP1 signaling governs the metabolic homeostasis in SCs, and boosting their activity is pro-survival, anti-inflammatory, and anti-oxidative, and thereby reduces neuronal mitochondrial dysfunction and suppresses neuronal hyperexcitability induced by chemotherapeutic agents. Our hypothesis is based on published data showing that PPARγ and LRP1 agonists prevent the development of pain-related behaviors after nerve damage and compelling new preliminary data showing that PPARγ and LRP1 regulate bioenergetics and mitochondrial dynamics in SCs in response to chemotherapeutics.
In Aim 1, we will examine the role of PPARγ and LRP1 in cell survival, mitochondria dynamics and heterogeneities in primary mouse SCs. We will examine ultrastructural and bioenergetic changes in nerve mitochondria in a tumor bearing oral cancer model treated with chemotherapeutics. In Aim 2, we will examine the role of SC PPARγ-LRP1 in chemotherapy-induced oxidative stress and neuroinflammation using primary mouse SCs and tumor bearing models of CIPPN. In Aim 3, we will examine the role of SC PPARg and LRP1 signaling in chemotherapy-induced changes in nociceptive behaviors, sensory neuron metabolism and hyperexcitability.
We will use two chemotherapeutics: paclitaxel and oxaliplatin for generalizability. All key findings from mouse studies will be validated in human cells and tissues including SCs, nerves collected from cancer patients, and novel SC-dorsal ganglion neuron cocultures. Abuse liabilities are determined by monitoring mouse behaviors. The potential effect of SC PPARγ-LRP1 signaling on tumor’s response to chemotherapeutics will be evaluated by monitoring tumor growth and histopathology. Proposed studies will validate the role of PPARγ in CIPPN, address new cellular and molecular mechanisms of PPARγ regulation of CIPPN, and identify LRP1 as a novel target for CIPPN. Clinical relevance is predicated on recent reports demonstrating that a selective PPARγ agonist is protective against nerve degeneration and inflammation in patients with rare neurological disorders. SP16, an innovative LRP1 agonist has shown safety and tolerability in clinical trials.
B, Remote Monitoring of Management of Chemotherapy induced Peripheral Neuropathy
The overall goal of this study is to demonstrate the efficacy of a new care model that facilitates symptom reporting and proactive treatment management to reduce CIPN symptoms.
Specific Aim1: Determine the efficacy of SCH-NP vs. usual care (UC) to reduce post chemotherapy CIPN symptoms over 12 weeks. We hypothesize SCH-NP will reduce CIPN symptoms measured on the Neuropathy Total Symptom Score 6 (NTSS6). 358 recently diagnosed CIPN patients will be randomized to 12 weeks of UC or SCH-NP care. Both groups will report symptoms daily via phone, app or website. The UC group will receive standard care through their treating physicians. In the SCH-NP group reporting moderate to severe symptoms will trigger NP call back to provide evidence-based protocolized treatment including medications, self-care strategies and referrals. Repeated measure analysis of the NTSS6 covariance will be used to compare groups.
Specific Aim 2: Determine the efficacy of SCH-NP vs. UC in improving CIPN specific quality of life and disability. We hypothesize that compared to UC, SCH-NP care will improve CIPN specific quality of life (EORTC CIPN 20) and reduce disability (CIPN-RODS) measured by repeated covariance analysis.
Specific Aim 3: Determine the impact of SCH-NP vs. UC on opiate usage and the utilization of available CIPN therapies. We hypothesize SCH-NP care will reduced opiate use measured by % of subjects on ≥ 50 MME/ day (high risk) and improve CIPN therapy utilization including neuropathic pain med use, time to prescription, selection of the correct therapy, adequate titration, max drug doses, and total number of CIPN treatments. This innovative study utilizes a novel technology based model to circumnavigate roadblocks in CIPN care.
The study’s broad impact and substantial significance derive from the model’s ability to deliver care to those in resource poor areas with reduced healthcare access in their time of greatest need.


